GMP / Validation
Smart Validation using Global Industry Trends to Ensure Safety, Quality and Efficacy in the Pharmaceutical Industry
Chiyoda’s technologies, developments, achievements and approach to new projects
Chiyoda is a forerunner in the field of engineering and constructing facilities for pharmaceutical validation, using our many years of experience to provide high-quality and sophisticated validation services for customers reflecting global industry trends, such as PIC/S GMP and Commissioning & Qualification (C&Q).
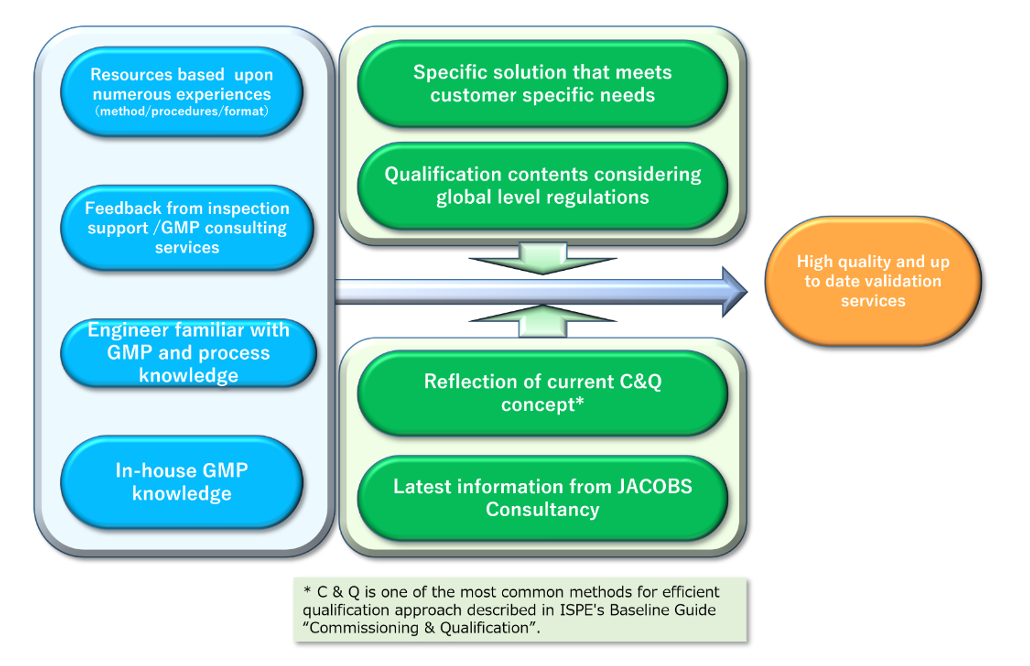
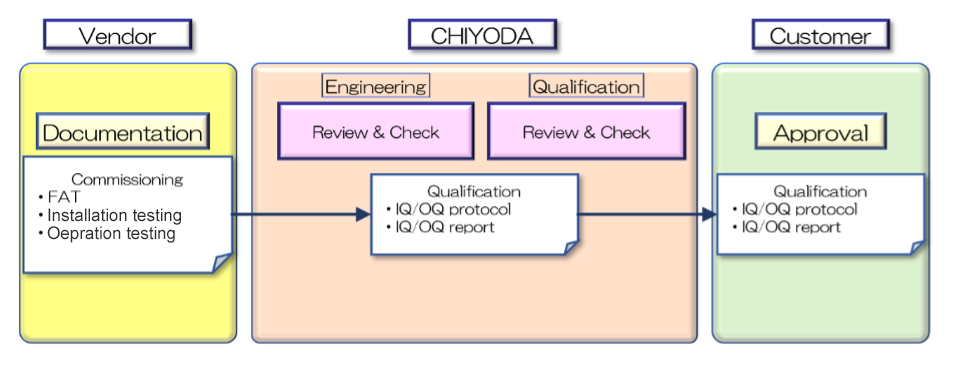
Cooperation with Engineering Teams for Efficient Validation
Validation is conducted through close cooperation between engineering and validation teams, reflecting global industry trends and effectively utilizing vendor and commissioning documentation.
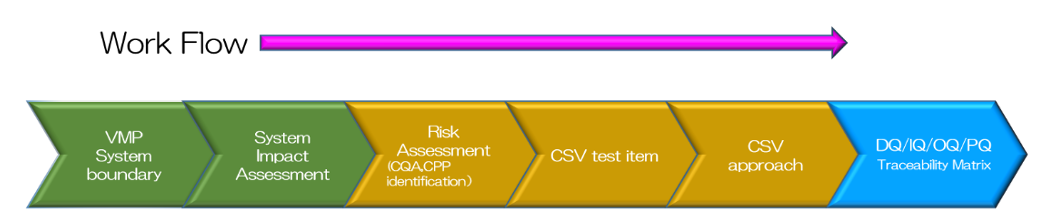
Efficient Computerized System Validation in accordance with Computer Systems
Chiyoda delivers efficient computerized system validation (stand-alone or embedded systems) by assessing the risks of the computer system and validation against increasingly stringent Data Integrity (DI) requirements and documenting the results.