Sterile Pharmaceuticals
Multi-purpose manufacturing line to meet CDMO needs by introducing advanced technology
Chiyoda’s technologies, developments, achievements and approach to new projects
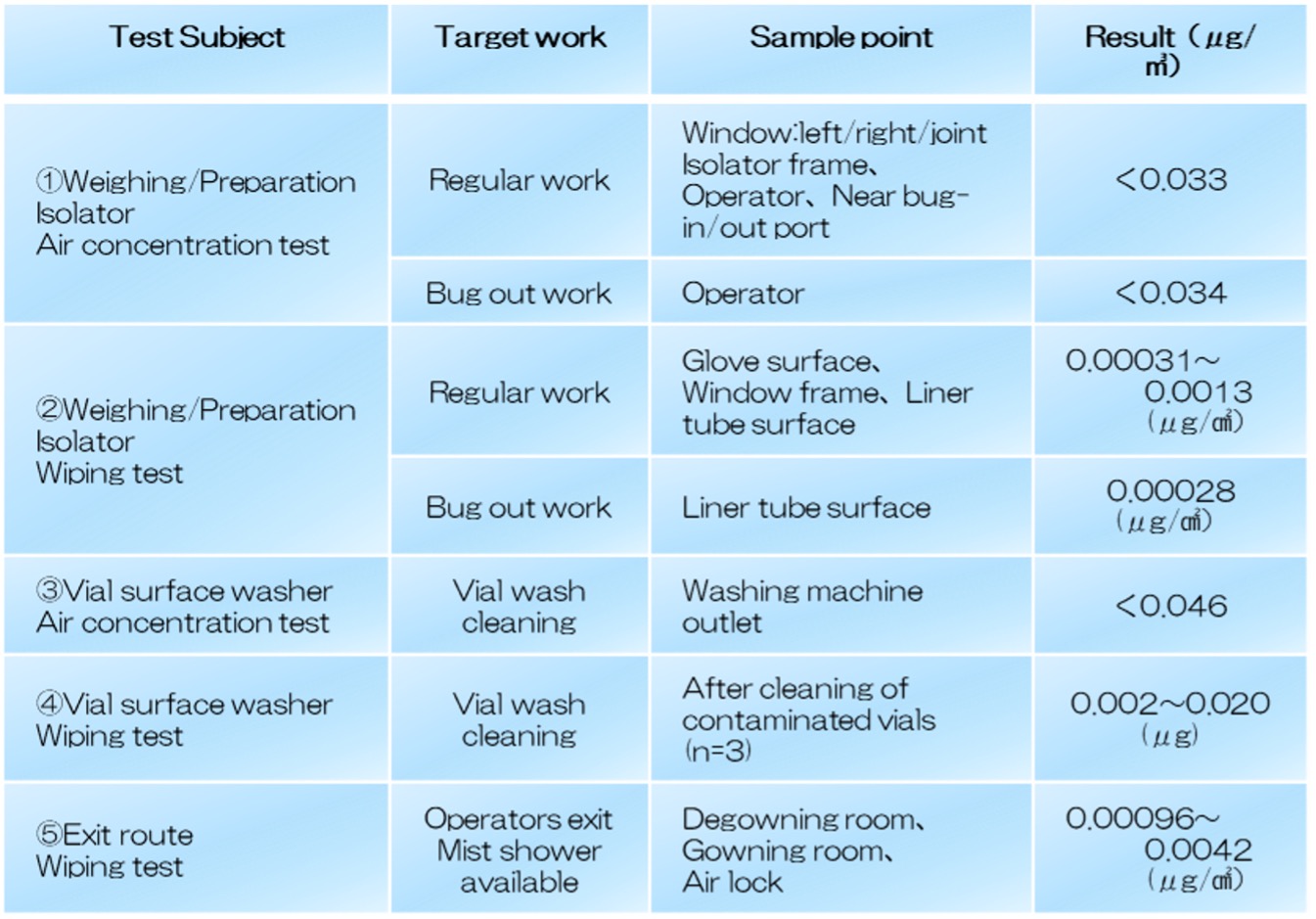
- Realization of High-Degree Sterility Assurance by Introducing Isolator.
- Containment Design Technology for Hazardous Substances while maintaining sterility.
- Enhanced equipment flexibility through effective implementation of single-use technology.
- Designing streamlined operations to save labor and energy costs for efficient small-batch, multi-product manufacturing.
- System construction integrating IoT.
A combination of various elemental technologies is required to achieve a high level of sterility assurance. It is necessary to establish manufacturing environment by isolators and HVAC, establish C/SIP for process equipment, and establish a barrier for foreign material contamination due to material handling, all of which must be easy to operate and allow for appropriate monitoring.
In recent years, there has been an increase in the number of cases in which multi-purpose production lines are required for contract manufacturing, such as the ability to manufacture various sterile products on the same line, rather than a dedicated production line for specific product.
We will offer proposals that will satisfy customer’s needs, such as safety design that takes into account the containment of hazardous materials, and the construction of highly flexible lines that effectively incorporates single-use technology in anticipation of future manufacturing plans.